by WL Bryden, DD Moore and S Shini, School of
Agriculture and Food Sciences, University of Queensland, Australia
First published in Milling and Grain, April 2015
Selenium exists in four oxidation states: elemental Se
(Se0), selenide (Se−2), selenite (Se+4), and selenate (Se+6) in a variety of
inorganic and organic matrices. The natural inorganic forms, selenite and
selenate, account for the majority of total global selenium.
Organically bound selenide compounds are predominantly
seleno-amino acids; the principle chemical form of Se in animal tissues is
selenocysteine, while selenomethionine predominates in plants.
The chemistry of selenium resembles that of sulphur in
several respects but these elements are not completely interchangeable in
animal systems.
Both, sulphur and Se occur in proteins as constituents of
amino acids. Sulphur is one of the most prevalent elements in the body and is
present in the sulphur-containing amino acids: methionine, cysteine,
homocysteine and taurine. Selenium is a trace element and a component of the
amino acids selenocysteine and selenomethionine. Selenocysteine is considered
the 21st amino acid in terms of ribosome-mediated protein synthesis.
Selenocysteine is identical to cysteine except that sulphur
is replaced by a Se atom, which is typically ionized at physiological pH.
The presence of selenocysteine in the catalytic site of
Se-dependent antioxidant enzymes enhances their kinetic properties and broadens
the catalytic activity of the enzymes against biological oxidants when compared
with sulphur-containing species. Selenocysteine (from animal tissues) and
selenomethionine (from plants) are both sources of selenium for synthesis of
SePs.
Replacement of selenocysteine by cysteine in a selenoprotein
usually results in a dramatic decrease of enzymatic activity, confirming that
the ionized selenium atom is critical for optimum protein function.
Biosynthesis pathway
Significantly, within all cell types there is a specific
biosynthesis pathway that facilitates selenocysteine synthesis and its
subsequent incorporation into SePs Cellular Se concentrations are therefore
tightly regulated. The regulation of selenoprotein synthesis is central to
understanding Se homeostasis and disorders following the failure of
homeostasis.
Cellular Se concentration is a key regulator of its
incorporation into SePs and acts mainly at the post-transcriptional level in
response to alterations in Se bioavailability. Selenocysteine biosynthesis
represents the main regulatory point for selenoprotein synthesis and not
absorption as occurs with many nutrients.
The biochemistry of Se is different from most other trace
elements as it is incorporated in proteins (SePs) at their highest level of
complexity and function. Selenoproteins incorporate selenium only in the form
of selenocysteine and this occurs during translation in the ribosome using a
transfer RNA specific for selenocysteine.
Seleno-amino acids (selenocysteine or selenocystine and
selenomethionine) are required for the synthesis of selenium-containing
peptides and proteins.
Importantly, selenomethionine (the major dietary organic
form of Se) that is biochemically equivalent to methionine, is not incorporated
into selenoproteins and therefore, is not a participant in the regulation of
selenium homeostasis. There are no known human or animal functionally active
SePs that contain selenomethionine.
Only proteins that are genetically programmed and perform
essential biological functions are classified as SePs. Some of these SePs are
enzymes such as the six antioxidant glutathione peroxidases and the three
thioredoxin reductases; the three deiodinases are involved in thyroid function
by catalysing the activation and deactivation of the thyroid hormones.
Some SePs have direct roles in modulating immunity and
reproductive function, while other SePs facilitate tissue distribution and
transfer of Se.
Selenoprotein P, for example, functions as a transporter of
selenium between the liver and other organs. The functional characterisation of
many SePs remains to be delineated.
Absorption, distribution and metabolic rate
An overview of the metabolism of Se is shown in Figure 1.
Absorption of selenium occurs in the small intestine, where
both inorganic and organic forms of Se are readily absorbed.
Selenite is passively absorbed across the gut wall, while
selenate appears to be transported by a sodium-mediated carrier mechanism
shared with sulphur.
Organic forms of Se are actively transported. The absorption
of selenomethionine is via the same carrier transport protein as methionine,
with competition taking place between methionine and its seleno analog.
Selenium is distributed throughout the body from the liver to the brain,
pancreas and kidneys.
The highest Se concentrations are found in the liver and
kidneys but the greatest total concentration occurs in muscle because of their
proportion of body weight. Selenium is transported by two SePs; selenoprotein P
and extracellular glutathione peroxidase (GSH-Px).
Other transport mechanisms have been postulated but not
delineated. Only insignificant transitory amounts of free selenomethionine are
found in blood. Following protein turnover, the released Se, can be recycled
via enterohepatic circulation or excreted. Selenium is eliminated primarily in
urine and faeces.
The distribution between the two routes varies with the
level of exposure and time after exposure.
In ruminants, selenite is the primary compound available for
absorption because the reducing conditions within the rumen convert the
majority of selenate to selenite.
In the rumen, about a third of selenite is converted to
insoluble forms that are passed into manure. Of the soluble selenite that reaches
the intestine, some 40 percent will be absorbed, compared to about 80 percent of
selenomthionine. As a consequence of these differences, in cows, the
digestibility of Se from selenite is around 50 percent compared to about 66
percent for selenium-yeast. There is no information on the impact of the gut
microbiota on the Se requirements of monogastric animals.
Inorganic Se is recognised by the digestive tissues and is
absorbed and converted into SePs.
In contrast, organic Se (selenomethionine) is not recognized
as Se-containing by mammalian cells. As a consequence, selenomethionine is
absorbed and metabolized relative to methionine needs.
If selenomethionine is broken down within the cell, Se is
released and recognized by the cell as a mineral. It is then processed
according to the need for Se.
However, if the cell does not break down selenomethionine,
it may be inadvertently incorporated into a wide variety of proteins that are
not genetically programmed to contain selenium.
The functionality of these proteins will be compromised. As
a metabolic safeguard, neither dietary selenocysteine nor selenomethionine is
directly incorporated into selenoproteins. All dietary forms of selenium must
be metabolised and converted to selenocysteine and selenoproteins under the
genetically controlled mechanism within the cell.
Much of the absorbed organic Se is transferred into the
amino acid pool, where together with the existing intracellular pool, it is
metabolised by different pathways (see Figure 1). From there, it is enzymatically
converted in the liver to selenide, which serves as the Se source for
selenocysteine synthesis.
Deficiency and requirements
Selenium acts biochemically in the animal or bird in a complimentary manner to vitamin E. Both nutrients prevent peroxidation of unsaturated fatty acids in cell membranes.
Most of the deficiency signs of these nutrients can be
explained by their antioxidant properties. The requirement for each is
therefore influenced by the dietary concentration of the other.
For example, the Se requirement of the chick is inversely
proportional to dietary vitamin E intake. Thus Se has sparing effect on the
requirement for vitamin E and vice versa.
Manifestation of Se deficiency can take many forms and
varies between species. Muscular degeneration or white muscle disease occurs to
varying degrees in all species. In birds, pancreatic fibrosis is an
uncomplicated Se deficiency, whereas exudative diathesis (generalised oedema
visible under the skin) is responsive to both Se and vitamin E.
Pigs with hepatosis diatetica (severe necrotic liver
lesions) are responsive to Se supplements, while both Se and vitamin E are
effective in treating mulberry heart disease (a dietetic microangiopathy).
Reproductive disorders, including retained placenta in dairy cows, and lowered
disease resistance are observed in all Se deficient species. Some species, such
as rabbits and horses, seem to be more dependent on vitamin E than Se for their
antioxidant protection.
This may reflect species differences in dependence on
non-selenium containing GSH-Px.
Selenium presents a nutritional conundrum because it is both
essential and highly toxic. There are several approaches to measuring Se
status. These include the measurement of changes in plasma Se concentration,
measurement of GSH-Px enzyme activity, and absorption/retention studies.
The use of stable isotopes of Se have been used in human
studies and to determine endogenous forms of selenium in foods. All of these
biomarkers are useful indicators of Se status but because of the role of Se in
many biochemical pathways, a single indicator may not be an appropriate index
of Se status.
Dietary supplementation
Selenium is routinely added to animal diets to ensure that
requirements are met.
There has been increased interest recently in Se dietary
supplementation to enrich animal products. The production of selenium-enriched
meat, milk and eggs is viewed as an effective and safe way of improving the
selenium status of humans.
There are a range of products available for dietary Se
supplementation (see Table 1).
Selenium is commonly added to diets as sodium selenite.
However, there has been growing interest in dietary addition
of organic Se. Organic sources are assimilated more efficiently than inorganic
Se and considered to be less toxic and therefore more appropriate as a feed
supplement.
Yeast has become the most popular vehicle for the addition
of organic Se because of its rapid growth, ease of culture and high capacity to
accumulate Se. The major product in selenized yeast is selenomethionine.
Selenomethionine was found to be four times more effective
than selenite in preventing the characteristic pancreatic degeneration caused
by selenium deficiency in chicks.
Selenium yeast (selenomethionine) was found to be much more
effective than inorganic Se in increasing the Se concentration of cow’s milk.
This is in accord with many animal studies and human clinical trials that have
demonstrated the superior efficacy of L-selenomethionine, in increasing Se
muscle content compared to inorganic Se.
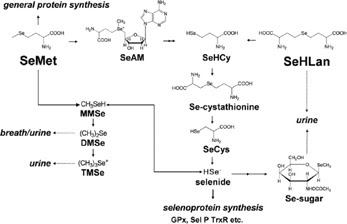 |
|
Figure 2. Proposed metabolic pathways for
SeHLan and SeMet in
animal cells (Source: Tsuji et al. 2010)
|
As shown in Figure 2, differences in
metabolism between SeHLan and selenomethionine may, in-part, explain the
apparent difference in toxicity.
Read the magazine HERE.
The Global Miller
Selenomethionie mimics methionine by sharing the same
metabolic pathways and can replace methionine in peptide synthesis, as noted
above, and thus disrupt protein synthesis.
As shown in Figure 2, the proposed metabolic pathway for
SeHLan appears to be much less complex; SeHLan is only utilised in the
trans-selenation pathway for selenoprotein synthesis and therefore is not
expected to interfere with the methionine metabolic pathways. The tissue
distribution of these two selenoamino acids may also contribute to differences
in toxicity.
Both are distributed throughout the body with higher liver
and pancreas accumulation of selenomethionine in contrast to SeHLan which
preferentially accumulates in the liver and kidneys.
At higher doses, selenomethionine has been shown to induce
pancreas damage whereas SeHLan is excreted by the kidneys without inducing
pancreatic damage.
Selenomethionine enriched yeast has been available
commercially for many years.
Recently, a yeast product enriched with SeHLan has become
available and a number of efficacy studies with growing pigs and broiler
chickens have been conducted in Australia with these selenoamino acid sources.
In the studies both selenomethionine (Sel Plex) and SeHLan
(AB Tor-Sel) were compared to sodium selenite. In the clean experimental
conditions, as demonstrated on many occasions, dietary supplementation with
both the inorganic and organic selenium resulted in similar animal and bird
performance.
However, tissue accumulation was significantly greater when
the organic forms of Se were fed, which is in accord with the literature.
Interestingly, the yeast enriched with SeHLan generated significantly higher Se
concentrations in muscle tissue than the selenomethionine enriched product.
The implication of this finding in both pigs and broilers
may imply a greater efficacy of SeHLan in stressful commercial environments.
Remarks
Selenium’s nutritional essentiality was discovered in the
1950s.
It is now clear that the importance of having adequate
amounts of Se in the diet is primarily due to the fact that this micronutrient
is required for the biosynthesis of selenocysteine as a part of functional
selenoproteins.
Although animals, and presumably humans, are able to
efficiently utilise nutritionally adequate levels of Se in both organic and
inorganic forms for selenoprotein synthesis, it is clear that the
bioavailability of Se varies, depending on the source and chemical form of the
Se supplement.
Tissue enrichment with Se is greater when organic forms of
the micronutrient are fed.
Organic selenium, in the form of yeast enriched with
selenomethionine, is widely used in animal nutrition.
Recently, yeast enriched with SeHLan became commercially available
and initial research suggests that it may be more efficacious than
selenomethionine for tissue accumulation of Se.
This has obvious implications for the production of Se
enriched animal products but may also be important in commercial production units.
Greater tissue reserves of Se may enhance an animals’ resilience to stress and
disease challenge.
A brief history of Selenium
Selenium (Se) is an essential trace element for animals and
humans. It was discovered in 1818 and named Selene after the Greek goddess of
the moon.
Selenium exerts its biological effects as an integral
component of selenoproteins (SePs) that contain selenocysteine at their active
site. Some 30 SePs, mostly enzymes, have been identified, including a series of
glutathione peroxidases, thioredoxin reductases and iodothyronine deiodinases.
The majority play important roles in redox regulation,
detoxification, immunity and viral suppression. Deficiency or low selenium
status leads to marked changes in many biochemical pathways and a range of
pathologies associated with defects of selenoprotein function may occur.
Selenium content of soils can vary widely.
In areas where soils are low in bioavailable Se,
deficiencies can occur in humans and animals consuming plant-based foods grown
in those soils.
Selenium deficiency have been reported in many countries
including China, Japan, Korea, and Siberia, Northern Europe, USA, Canada, New
Zealand and Australia. Within each country there are large regional differences
in soil Se status and in some localities there are plants that accumulate Se
resulting in selenosis or Se toxicity to grazing animals.
Dietary Se supplementation was first permitted some 40 years
ago.
Since then, there has significant advances in our knowledge
of Se metabolism and the important role that Se plays in animal productivity
and health.
During this period, Se has become an important addition to
dietary supplements for animals.
Further reading
Bellinger FP, Raman AV, Reeves MA, Berry MJ. 2009.
Regulation and function of selenoproteins in human disease. Biochemical
Journal, 422:11-22.
Brennan,KM, Crowdus, CA, Cantor, AH. et al 2011 Effects of
organic and inorganic dietary selenium supplementation on gene expression
profiles in oviduct tissue from broiler-breeder hens Animal Reproduction
Science 125: 180– 188
Celi P, Selle PH, Cowieson AJ. 2014. Effects of organic
selenium supplementation on growth performance, nutrient utilisation, oxidative
stress and selenium tissue concentrations in broiler chickens. Animal
Production Science 54, 966–971.
Fairweather-Tait SJ, Collings R. Hurst, R. 2010. Selenium
bioavailability: current knowledge and future research requirements. American
Journal of Clinical Nutrition, 91:1484S-1491S.
Kumar BS and Priyadarsini KI. 2014 Selenium nutrition: How
important is it? Biomedicine & Preventive Nutrition 4: 333–341
Schrauzer GN, Surai PF. 2009. Selenium in human and animal
nutrition: resolved and unresolved issues. Critical Reviews in Biotechnology.
29:2-9.
Tsuji Y, Mikami T, Anan Y, Ogra Y. 2010. Comparison of
selenohomolanthionine and selenomethionine in terms of selenium distribution
and toxicity in rats by bolus administration. Metallomics. 2:412-418.
This blog is maintained by The Global Miller staff and is supported by the magazine GFMT
which is published by Perendale Publishers Limited.
For additional daily news from milling around the world: global-milling.com




I appreciate your great work that will make the world healthy and fit, thanks!
ReplyDeleteReally an awesome blog that suggest to the Supplements, it will really be healthy for the people who want to be fit and healthy.
Supplements